F.D.A. Approves Pfizer’s R.S.V. Vaccine for Older Adults
The News
The Food and Drug Administration on Wednesday approved Pfizer’s vaccine against the respiratory syncytial virus, or R.S.V., for adults age 60 and older, the second approval granted for shots offering protection from the virus this month.
GSK was the first drugmaker to get the F.D.A.’s permission to market an R.S.V. vaccine on May 3. The vaccines are expected to be available in the fall before the winter R.S.V. season.

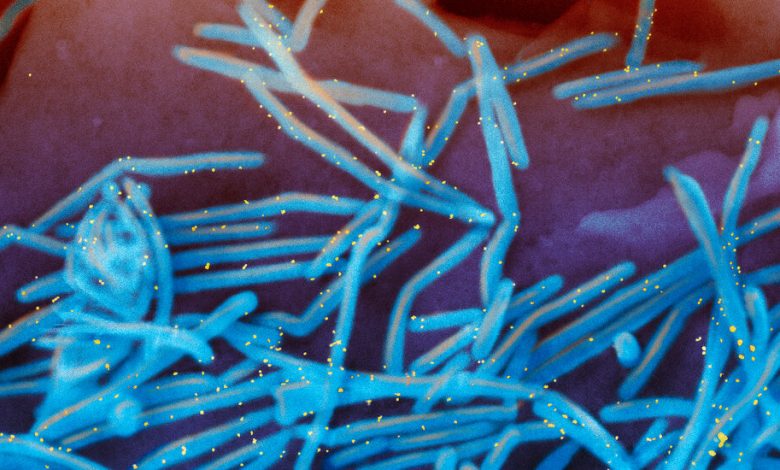
A colorized electron microscope image of the human respiratory syncytial virus, with virions in blue and antibodies in yellow, shedding from the surface of human lung cells.Credit…National Institute of Allergy and Infectious Diseases, via Associated Press
Why It Matters: Older Adults Are at Higher Risk.
Each year, about 60,000 adults 65 and older are hospitalized with R.S.V. and about 6,000 to 10,000 die from the virus, the F.D.A. estimated. The Centers for Disease Control and Prevention estimated that in one year, more than 21,000 people in that age group would need to take the GSK vaccine to prevent one R.S.V. death; the number was nearly 25,000 for the Pfizer shot.
The virus was a key driver in the winter’s “tripledemic” of Covid, flu and R.S.V. that was particularly hard on young children, resulting in overwhelmed hospitals.
Infants and toddlers are also at elevated risk; R.S.V. is considered a leading killer of infants globally. Several treatments, including a maternal vaccine and a monoclonal antibody for infants against R.S.V., are under agency review.
Background: Advisers Aired Safety Concerns.
During an advisory meeting on March 1 about both vaccines, doctors reviewed detailed data provided by the drugmakers.
Pfizer’s product, called Abrysvo, proved nearly 67 percent effective against cases of the virus with two symptoms and 86 percent effective against cases with three or more symptoms, according to data submitted to the F.D.A. The GSK vaccine, called Arexvy, was nearly 83 percent effective against severe R.S.V.
But the advisory panel also raised concerns about a few cases in which vaccine recipients developed autoimmune syndromes shortly after receiving the shots.
In a Pfizer study of about 34,000 patients who received the R.S.V. vaccine, a week after the shot, one patient developed a life-threatening case of Guillain-Barré syndrome, a condition where the immune system attacks the nervous system. A second patient developed a subtype of that condition called Miller Fisher syndrome eight days after receiving the shot.
Those cases put the incidence rate of the condition at about one in 9,000 — though they are typically seen at a rate of about one in 100,000 in the general population. Some advisers, also noting the low incidence of severe R.S.V. in the patient pool, found those numbers troubling. The final vote of the F.D.A.’s advisory panel in favor of the Pfizer vaccine’s safety and efficacy was 7 to 4. The panel voted 10 to 2 in favor of the GSK vaccine, which was also linked to one Guillain-Barré case and two others of a possibly related disorder.
What’s Next: When Will the Shots Be Available?
C.D.C. advisers are expected to discuss recommendations to health care providers about the shots in a meeting next month. So far, they have signaled that the data from the GSK and Pfizer trials support use of the vaccines in people ages 65 and older.
A Pfizer spokeswoman, Jerica Pitts, said the company was ready to ship the vaccine. She did not know the price of the vaccine but said there would be no co-pay for vaccines deemed medically necessary under Medicaid and Medicare. GSK said earlier that its vaccine would be available in the fall.




